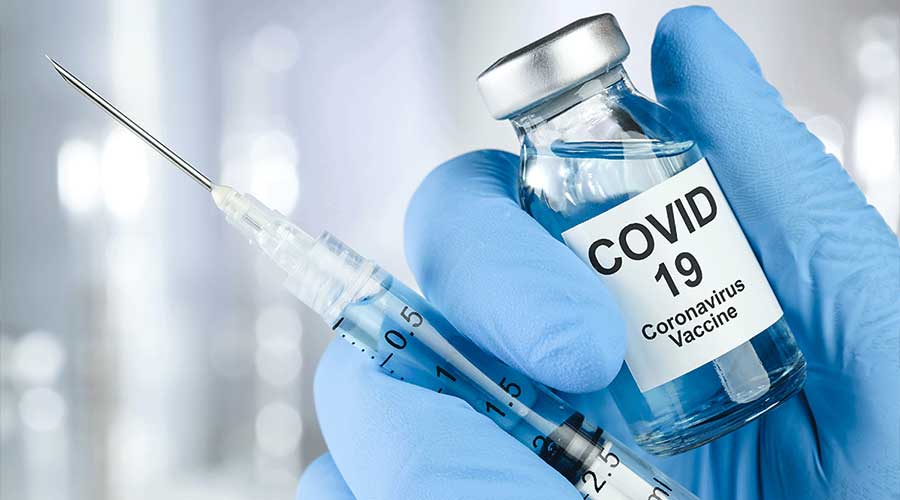
Drug Regulatory Authority of Pakistan (DRAP) has given formal approval to the Ministry of NHSRC for Phase III clinical trials of the COVID-19 vaccine.
The statement was given by the NIH, “The formal approval from DRAP for Phase III Clinical Trial of Recombinant Novel Coronavirus Vaccine Adenovirus Type 5 vector (Ad5-nCoV) developed by CanSinoBio and Beijing Institute of Biotechnology China [BIB]. This will be the first-ever phase III clinical trial for any vaccine in Pakistan.”
Read more: Who would be the first to get a Covid-19 vaccine?
The approved 3rd phase for the clinical trial of the COVID-19 vaccine has been developed by the Cansino Biologists and Beijing Institute of Biotechnology. Cansino Bio has launched in Russia, China, Argentina, and Chile that will soon be launched in Saudi Arabia as this is a multinational multi-center clinical trial, said a spokesman for the National Institutes of Health.
Moreover, Major General Amir Ikram, Executive Director, NIH, Islamabad is the Principal Investigator of Multi-Center Clinical Trials in Pakistan. The Phase III testing of Covid-19 vaccine in Pakistan is a multilateral activity among the National Institutes of Health, Cansino Bio company, and A. J. M Pharma.
This study will be conducted at medical research centers of the country including Aga Khan Medical University Karachi, Indus Hospital Karachi, Shaukat Khanum Memorial Hospital Lahore, Shifa International Hospital Islamabad, and UHS Lahore.
Pakistan’s ability to develop vaccines locally will be enhanced in this way if the phase III clinical trial in Pakistan initiates also the country would have a positive impact and image around the world. The vaccine would be supplied at reasonable prices across the country.
However, this is the first time that some company is conducting a clinical trial of COVID-19 vaccine on humans, Casino Bio company became the first-ever company to do so. The company published its Phase 1 trial results in May 2020 and Phase 2 results in July 2020.
The National Institutes of Health, Pakistan will be on the list of priority countries that will have early access to the coronavirus vaccine.
Image source