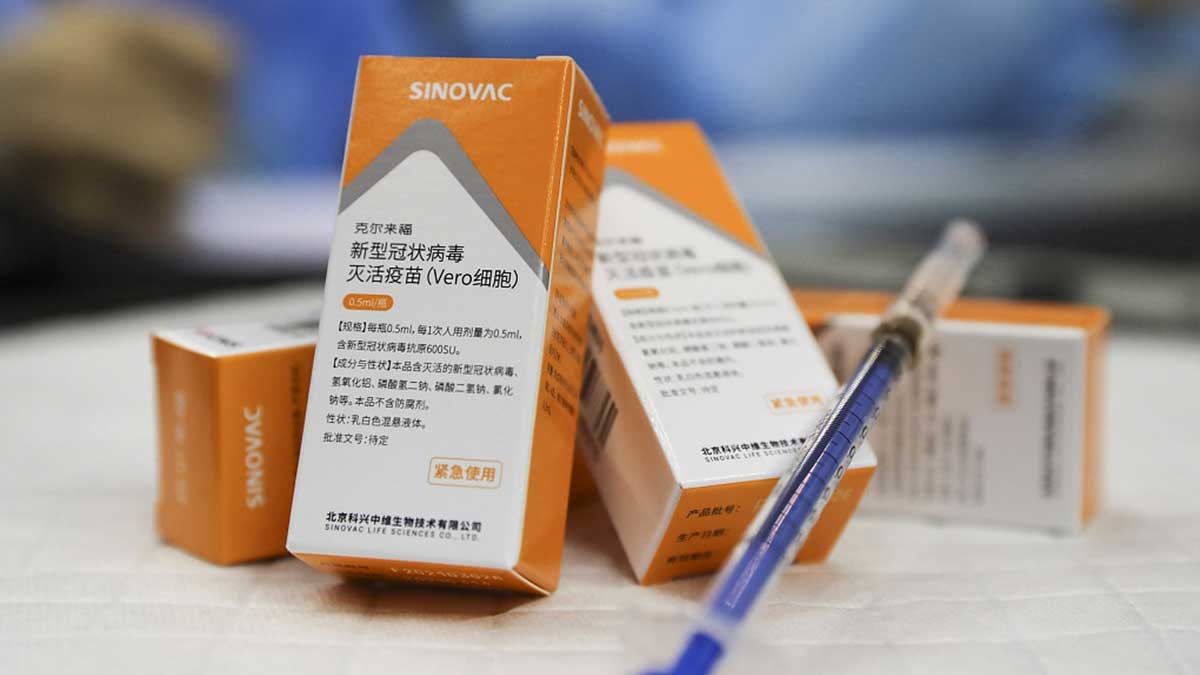
The World Health Organization approved the Sinovac Covid-19 vaccine for emergency use on Tuesday, making it the second Chinese vaccine to do so.
“WHO today validated the Sinovac-CoronaVac Covid-19 vaccine for emergency use, giving countries, funders, procuring agencies and communities the assurance that it meets international standards for safety, efficacy and manufacturing,” the UN health agency said in a statement.
A WHO emergency listing informs national regulators about the safety and effectiveness of a product. It will also permit the vaccine to be included in COVAX, a worldwide programme to give vaccines mostly to developing nations that is experiencing serious supply issues due to an Indian export ban.
Read more: 500,000 SinoVac coronavirus vaccine doses arrive in Pakistan
In a statement, the independent panel of experts recommended Sinovac’s vaccination for individuals over the age of 18, with a second dose given 2-4 weeks later. There was no upper age limit because research indicated that it may have a protective impact on the senior citizens.
The move was taken after the WHO’s technical advisory panel reviewed the most recent clinical data on the Sinovac vaccine’s safety and efficacy, as well as the company’s manufacturing practises, which began meeting on May 5.
It is the second Chinese-made vaccine to be approved by the WHO to combat COVID-19, following the approval of a shot made by state-backed Sinopharm on May 7.
CanSino Biologics, a third Chinese vaccine manufacturer, has submitted clinical trial results, but no WHO evaluation has been arranged.
As of the end of May, Sinovac announced it has distributed over 600 million doses of its vaccine both domestically and internationally, with over 430 million doses delivered.
Sinovac Shot Seen Highly Effective in Real World Study
According to the WHO, vaccination effectiveness results showed that the vaccination prevented symptomatic disease in 51% of those immunised and severe COVID-19 and hospitalisation in 100% of the study population.
In a previous evaluation paper, the WHO’s separate Strategic Advisory Group of Experts (SAGE) stated that vaccine efficacy in multi-country Phase III clinical trials ranged from 51 percent to 84 percent.